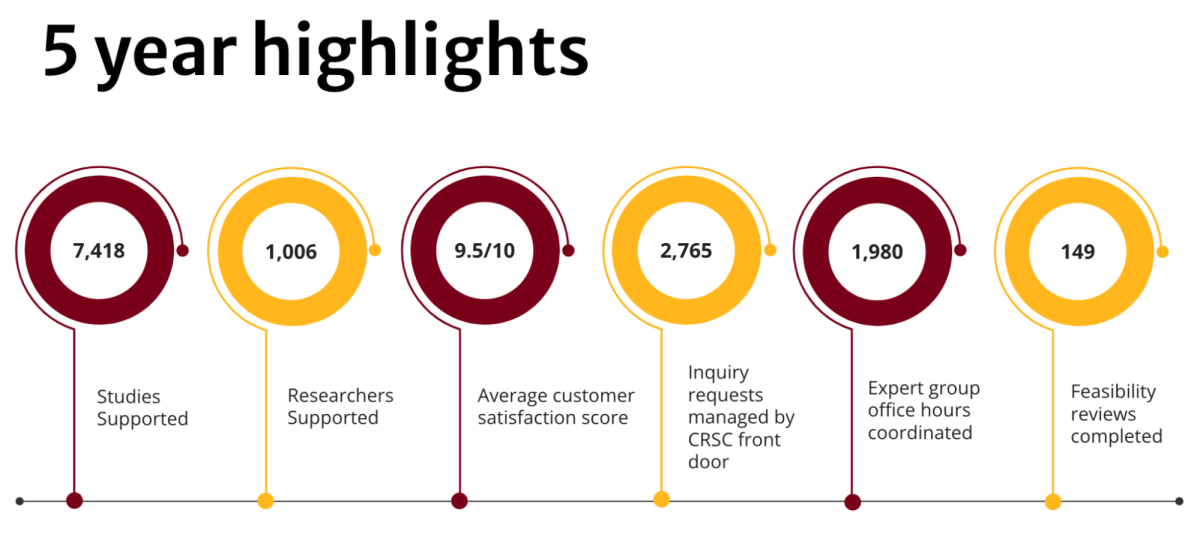
In the last five years, the Clinical Research Support Center has been dedicated to positioning University of Minnesota research teams for success. The center unites clinical research experts from 20 distinct units across the University and the broader health center to help investigators and study teams conduct clinical research more efficiently and effectively.
The CRSC first opened its doors in September 2018, as part of a collaboration among the Clinical and Translational Science Institute, the Office of the Vice President for Research, Fairview Health Services, and University of Minnesota Physicians.
Since then, it has helped thousands of University researchers and study teams enhance their protocols, navigate the clinical research process, and get their studies started swiftly.
To celebrate the CRSC’s fifth anniversary, we’re highlighting five ways it has helped PIs and study teams.
1. Providing tailored guidance
At the CRSC, support is customized to each researcher's study needs. The center meets researchers where they are in the process, quickly surrounding them with the expertise needed to advance their study.
This was the experience of Jessica Nielson, PhD, an assistant professor in the Department of Psychiatry and Behavioral Sciences who reached out to the CRSC for help with her project. Her study sought to better understand how psilocybin changes the brain, with a goal of finding a way to help those who suffer from conditions such as depression, addiction, and post-traumatic stress.
“This was my first clinical research study, and I can’t imagine getting it up and running without the CRSC,” says Dr. Nielson. “Both my FDA and IRB applications went very smoothly and were approved quickly because the CRSC experts I worked with thought of everything that needed to be addressed long before I submitted my protocol.”
The support helped position Dr. Nielson for success, and her study cleared all the necessary approvals.

2. Supporting study start-up
The 20-plus support teams part of the CRSC are a critical resource for University researchers who need to get their studies designed, set up, and launched.
For example, in the early days of the Covid-19 pandemic, the Masonic Cancer Center Clinical Trials Office (MCC CTO) and the CRSC successfully translated a Phase 1 cell therapy trial for COVID-19 treatment from idea to FDA and Institutional Review Board (IRB) approval to site initiation visit (SIV) in just 45 days.
“That start-up timeline is one for the record books”, said Jeff Miller, MD, Deputy Director, Masonic Cancer Center.
By working through the CRSC, researchers had a simple way to access experts from across UMN and the broader health center. Experts from CTSI helped with all aspects of initiating the study in collaboration with the MCC CTO, providing support for study planning and coordination, biostatistics, financials, IRB submissions, regulatory issues, and more. Through the CRSC, the team could also tap into experts from the UMN Sponsored Projects Administration (SPA), the IRB, and Fairview Health Services.
With the CRSC’s support, study start-up activities happen in parallel, and account for the necessary aspects of clinical trials initiation. It’s this cohesive, collaborative approach that significantly shortened the interval from idea to implementation.
3. Improving study design
Feasibility reviews are a signature, award-winning service offered through the CRSC. The free service provides University investigators with efficient, comprehensive protocol reviews by clinical research experts.
According to a study published in the Journal of Research Administration, the feasibility review process improves the quality of human subjects research protocols and better prepares them for IRB approval.
The study, conducted by CTSI faculty and staff, detailed the feasibility review process and examined 116 studies across eight of the University’s schools and colleges. It found the process resulted in submissions that received one or fewer protocol-related stipulations from the IRB and led to an investigator satisfaction rating of 4.71 out of 5, on average.
This was the experience of Niloufar Hadidi, PhD, APRN, CNS-BC, FAHA, an associate professor with the School of Nursing, who turned to the CRSC for her study that aims to reduce stroke rates in Black communities. Guidance from the feasibility review helped reduce the number of protocol-related IRB stipulations, getting swift approval for the study’s first phase and ultimately streamlining the study’s early steps.

4. Fostering a culture of innovation and process improvement
Over the years, the CRSC has worked hard to create a culture of innovation and process improvement. Team members continuously seek feedback from each other and the research community to determine where they can make a difference, and they’ve embarked on numerous process improvement projects.
For example, the CRSC co-facilitated a collaborative project that sought to add clarity to the ancillary review process. It brought together stakeholders from across the University clinical research community, and the effort led to the creation of several ancillary review resources available on the Human Research Protection Program (HRPP) website.
In addition, CRSC team members have actively disseminated what they’ve learned, to help other teams and institutions across the country. In addition to publishing a manuscript in the Journal of Research Administration, CRSC colleagues have presented seven posters, two of which won national awards: One on IRB stipulations and another on the feasibility review process.
5. Connecting researchers to resources
In addition to the thousands of studies supported, the CRSC has fielded more than 2,760 inquiries through its main email address and phone line. The CRSC is committed to helping to connect researchers to the experts, services, and guidance they need, when they need it.
To do this, the CRSC builds upon strong working relationships within and across teams and embraces a culture of learning, driven to learn from one another to better serve the research community.
“It’s really helpful to have someone hold my hand as we walk through the process together,” Elizabeth Lusczek, PhD, an assistant professor with the Medical School, explained in a video interview. “Without the CRSC, I would still be wondering who to talk to, who I needed to be put in touch with to get the information I needed.”
To request support from the CRSC’s clinical research experts for your study, contact [email protected] or 612-625-4000.