About CTSI
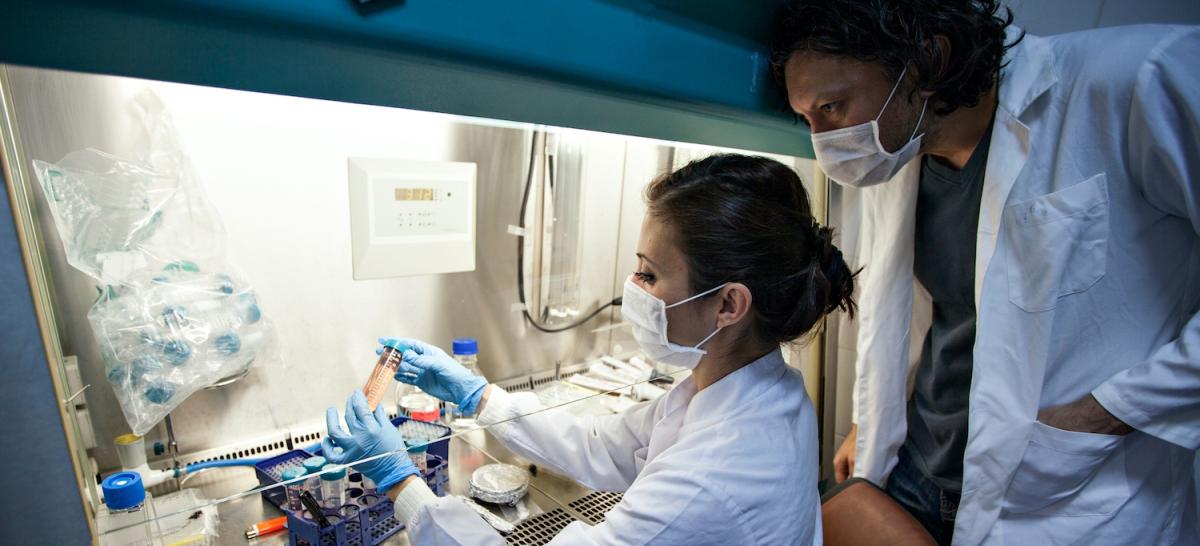
The Clinical and Translational Science Institute (CTSI) at the University of Minnesota is enhancing the way research is conducted to make a meaningful impact on people’s lives.
CTSI is part of the prestigious Clinical and Translational Science Award (CTSA) program, which aims to get interventions to patients and populations more quickly and enable research teams to tackle system-wide problems in clinical and translational research.
Serving the UMN research community
Training
Career development programs and other training opportunities for faculty, staff, trainees, students, and community members
Contributing to the national CTSA network
CTSI contributes to and collaborates with the national CTSA consortium. We are one of more than 50 CTSA program hubs working together to speed the translation of research discovery into improved patient care.
National collaborations
Programs and initiatives
How we’re supported
The Clinical and Translational Science Institute (CTSI) at the University of Minnesota's (UMN) is supported through the National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program. We also receive support from the University of Minnesota.
Making a difference
We’re dedicated to advancing discoveries that improve the health of individuals and the public.