Susan Everson-Rose, PhD, MPH, had an idea that could help heart attack survivors, but needed support making the jump from concept to pilot study to major grant application.
Through CTSI, she became connected to a network of clinical research specialists, who mapped out a plan and provided ongoing guidance for initiating the study, developing a protocol, recruiting participants, and more.
“Our vision is to streamline the study start-up process and ease the research journey for study teams by giving them a simple way to access a wide range of resources,” says Timothy Schacker, MD, a CTSI Associate Director who oversees the institute's clinical and translational research services. “This gives study teams the support they need to develop strong, feasible protocols that are well-positioned to lead to meaningful results, secure major grants, and become published by scientific journals.”

Lifting the research project off the ground
Dr. Everson-Rose began collaborating with CTSI on the project upon receiving a CTSI pilot grant that would support planning efforts for her study, which sought to examine how a mindfulness-based stress reduction program affected heart patients.
Dr. Everson-Rose turned to CTSI’s clinical research specialists for guidance throughout the duration of her study. CTSI identified and addressed potential study feasibility issues, provided ongoing consultations, joined weekly team meetings, and connected her with other resources along the way.
To put the project on the right track, CTSI developed a checklist that mapped project tasks and milestones to a timeline that would take the study from startup through regulatory approvals and to enrollment.
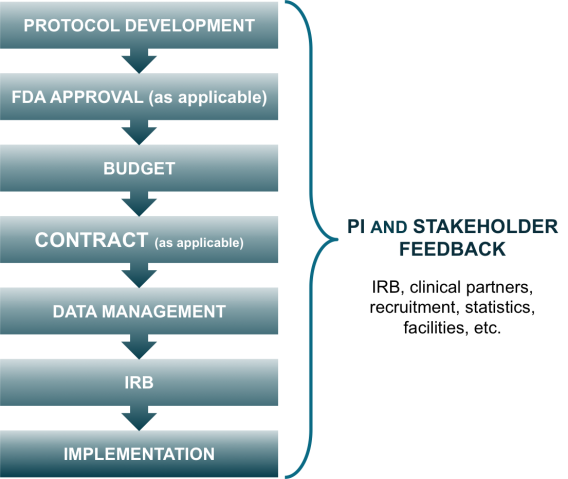
"A clear, detailed project checklist combined with Dr. Everson-Rose’s highly engaged, team-based approach kept the study organized and on-track, and helped maintain momentum throughout the project,” says Stacy Valenzuela, a former CTSI clinical research specialist who had supported the study.
CTSI also assisted with the budget and reviewed the protocol, filling in gaps where needed -- such as in areas regarding monitoring procedures, data management, and statistics.
Receiving input from clinical research specialists was especially important to Dr. Everson-Rose, who is an accomplished epidemiological researcher, but had never attempted a clinical trial.
“CTSI helped me understand what needed to be done to lift a clinical study off the ground, and navigate the clinical research environment,” says Dr. Everson-Rose. “As someone who is new to the complex world of clinical trials, emphasizing education and guidance is incredibly valuable.”
Surrounding the project with research specialists
As the study progressed, CTSI clinical research specialists connected Dr. Everson-Rose with other services, including those that could offer guidance on recruitment, biostatistics, informatics, and regulatory issues.
For example, CTSI recruitment specialists supported the study by drawing from their experiences helping hundreds of University studies attract participants. They joined regular team meetings, advised on recruitment strategies, and edited the copy used in participant brochures.
The study also received data management and statistical guidance from CTSI biostatisticians, and support from CTSI regulatory specialists during the Institutional Review Board (IRB) submission process.

CTSI also advised on the staffing needed to adequately support the study’s implementation, which received financial support from other University of Minnesota units. Specifically, the study received funding from the Office of the Vice President for Research’s Grant-in-Aid program and two divisions within the Medical School’s Department of Medicine: Cardiovascular and General Internal Medicine.
Hitting recruitment goals
Attracting enough participants is challenging for studies nationwide, plus Dr. Everson-Rose’s pilot trial had its own set of obstacles. Specifically, they would be having several patients attend sessions at the same time, and requesting a significant time commitment from individuals who had just experienced a traumatic coronary event or procedure.
In addition to regularly engaging with recruitment specialists, Dr. Everson-Rose and team brought in CTSI informatics consultants who could help access the electronic medical records of more than 2.5 million Fairview Health Services patients who consented to share their health records for research purposes.
In what Dr. Everson-Rose called a “seamless” and “efficient” process, informatics specialists helped find the most eligible patients for the study, and worked with Fairview to mail letters inviting these patients to consider participating.

These tactics, combined with a collaboration with a Fairview Southdale Hospital cardiovascular rehab therapist, helped the study hit its recruitment goals by its deadline, and exceed retention goals.
The study team aimed to retain 92 percent of patients throughout the duration of the ten-month study, yet ultimately retained more than 95 percent at the three-month mark and 98 percent at the six-month mark. Dr. Everson-Rose considers this to be “phenomenally successful”, noting that once participants committed to the study, they became very committed.
Saving money through electronic consents
Upon joining the study, participants could complete the consent form and study questionnaires from anywhere – not just the clinic room, where participants traditionally fill out these forms.
This was possible because Dr. Everson-Rose’s team used eResearch, an electronic consenting tool that CTSI was developing and wanting to better understand its usability and feasibility.
All participants consented via eResearch, with more than two-thirds consenting remotely before their first visit. This enhanced the entire process, as Dr. Everson-Rose explains:
“The eResearch electronic consenting tool was efficient and easy-to-use for participants, and resulted in significant savings on clinic space and staff. Each time a participant opted to complete the consent form and questionnaires from home, we’d save approximately $114. This ultimately led to more than $3,400 in savings over the course of our study.”
Pursuing a fully powered clinical trial
The pilot trial was successful, thanks to the hard work and dedication of the study team combined with the ongoing support they received.

Dr. Everson-Rose considers this to be a team effort, noting that CTSI played a key role in helping the study team take the trial’s critical early steps:
“CTSI provided layers of support in a seamless way, which helped position our study for success. Specialized guidance helped us hit our enrollment goals on-time, exceed our retention goals, and efficiently manage our budget – all accomplishments that helped strengthen the NIH application we recently submitted.”
CTSI clinical research specialists continued to be involved as the study transitioned from planning to implementation and, later, the National Institutes of Health (NIH) application process.
CTSI helped with various sections of the NIH application, such as the budget, protocol details, and statistical plan. Plus, the trial proposes continuing to use eResearch for electronic consenting and questionnaires, given the tool’s budget- and participant-friendly benefits.
In addition to pursuing a larger grant, the study team published an abstract of the pilot study results in Psychosomatic Medicine and presented their findings via a poster at the American Psychosomatic Society annual meeting.
Creating a better future for heart patients
As reported in the Minnesota Daily, should the project become funded, it would enable Dr. Everson-Rose to pursue a fully powered clinical trial. This trial would have more funding and more participants, to rigorously examine how a mindfulness-based stress reduction course affects survivors of heart attacks and other coronary events and procedures.
“Getting our pilot study off the ground was a critical first step in exploring whether such a study would be feasible,” says Dr. Everson-Rose. “By advancing our project along the clinical research trajectory, our hope is to unearth new strategies for improving heart patients’ quality of life.”
